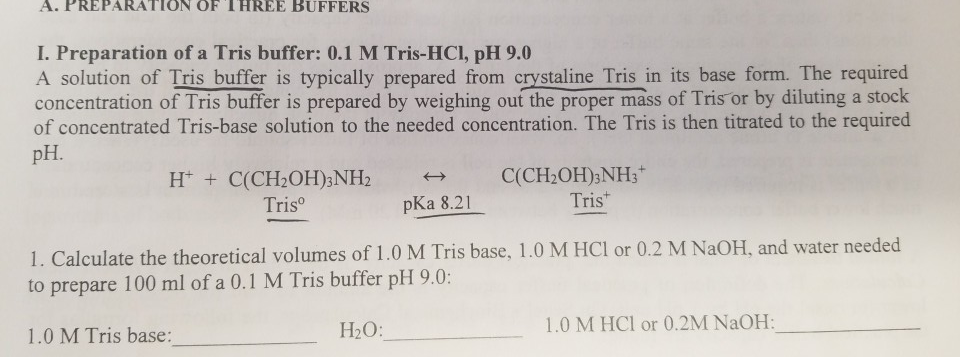
SOLVED: Solution #1: 500. mL of 150 mM Tris, pH 8.50 You have the following available Tris base (FW = 121.1 g/mol) pKa 8.10) 1.0 M HCI Solution #2: 750. mL of

WO2014165660A1 - Pharmaceutical formulations for subcutaneous administration of furosemide - Google Patents

Measurement of pHT values of Tris buffers in artificial seawater at varying mole ratios of Tris:Tris·HCl | Semantic Scholar

SOLVED: Text: Show the calculations for how you would prepare each of the following buffer solutions Solution #1: 500 mL of 150 mM Tris, pH 8.50 You have the following available: Tris
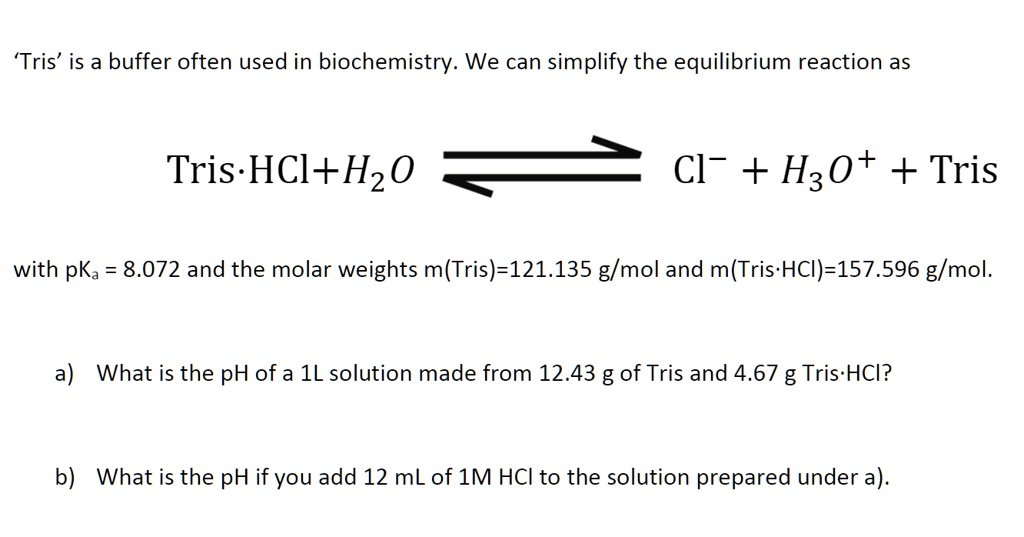
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar





![BT156] 2M Tris-HCl, pH 9.5 | Biosolution BT156] 2M Tris-HCl, pH 9.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/07/BT066-Tris-HCl.jpg)